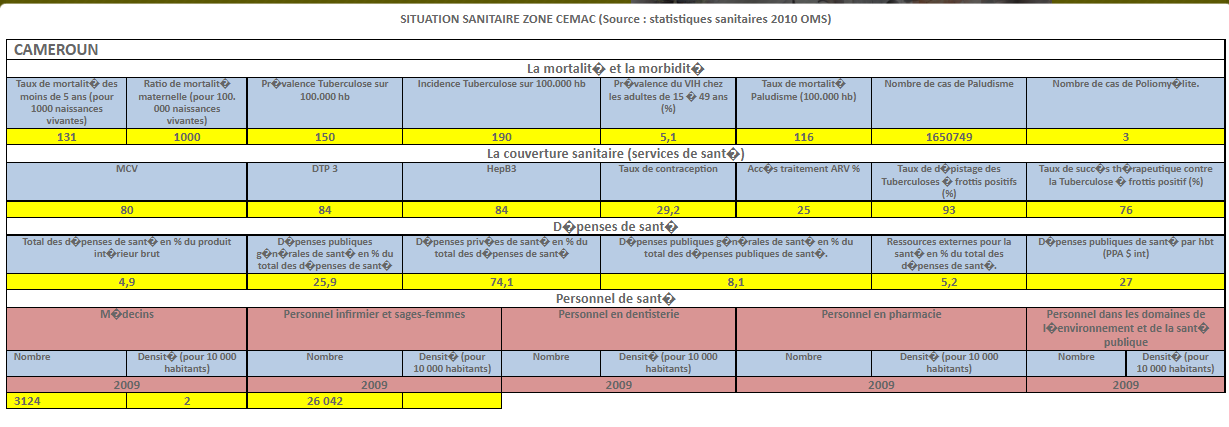
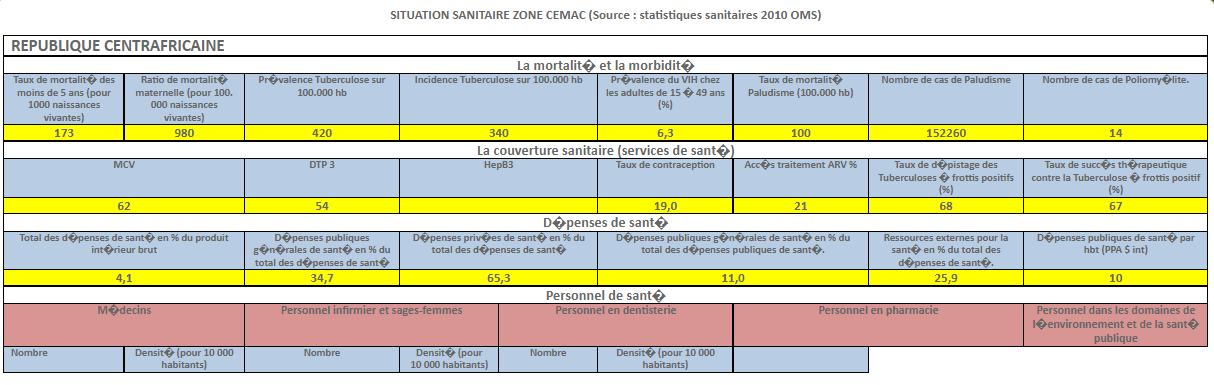
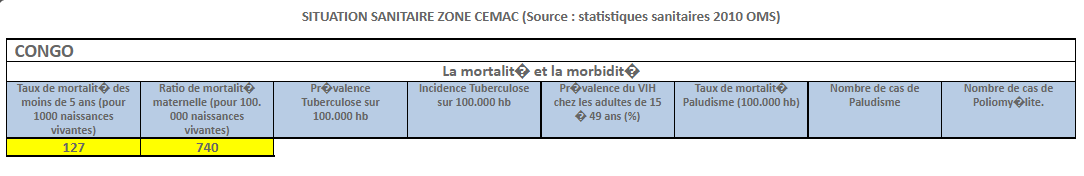
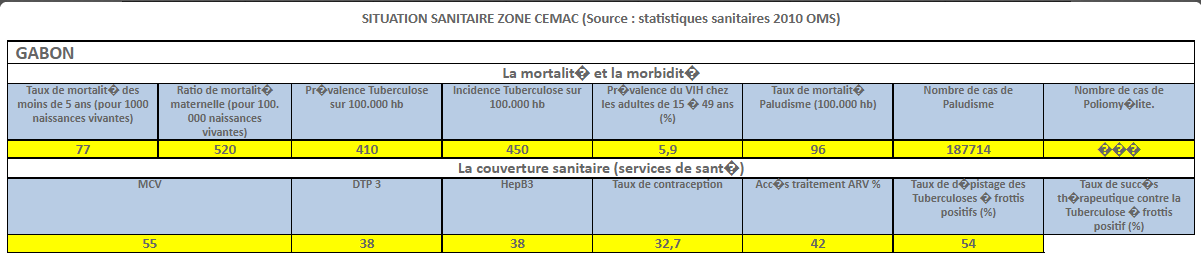
Harmonization of National Pharmaceutical Policies in Central Africa
The harmonization of pharmaceutical policies consists in bringing into agreement the various regulatory texts and practices that govern pharmacy with a view to opting for an identical and common line of conduct in the countries of the sub-region.
- to harmonize national pharmaceutical policies and existing practices;
- to adopt the approval procedures common to the six States;
- to improve drug procurement procedures;
- to set up a common price policy;
- to promote the development of human resources in the field of pharmacy and medicine.
Partners:
- WHO/Afro
- EU
- Cameroon Ministry of Health
- French Ministry of Foreign and European Affairs (French Cooperation)
- National Councils of Orders of Pharmacists
- NEPAD
As part of the CEMAC/France partnership, a technical adviser, of French nationality, has been available to the project team since September 2011. The terms of reference include three major projects: this involves supporting the OCEAC within the framework of the project to set up common pharmaceutical regulations in the sub-region, and in particular on the harmonization component of national drug registration systems, the preparation of a special meeting on counterfeit drugs and the creation of a sub-regional faculty of pharmacy.
- Participation of OCEAC/HPPN in the AFSSAPS Franco-African Meeting 2012 (March 19 and 20, 2012, Paris)
- Interest of a regional pharmaceutical product quality control laboratory in the CEMAC zone
- FOURTH MEETING OF PHARMACY AND MEDICINE DIRECTORS OF CENTRAL AFRICA
- 2nd MEETING OF NATIONAL PHARMACY AND MEDICINE OFFICIALS
COMMON PHARMACEUTICAL POLICY DRAFT – Proyecto POLITICA FARMACEUTICA COMUN – COMMON PHARMACEUTICAL POLICY Project