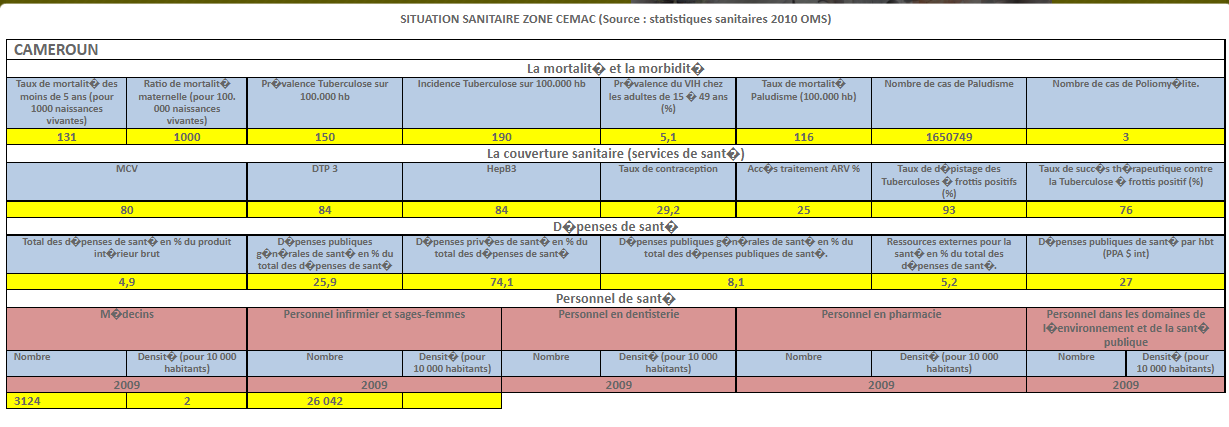
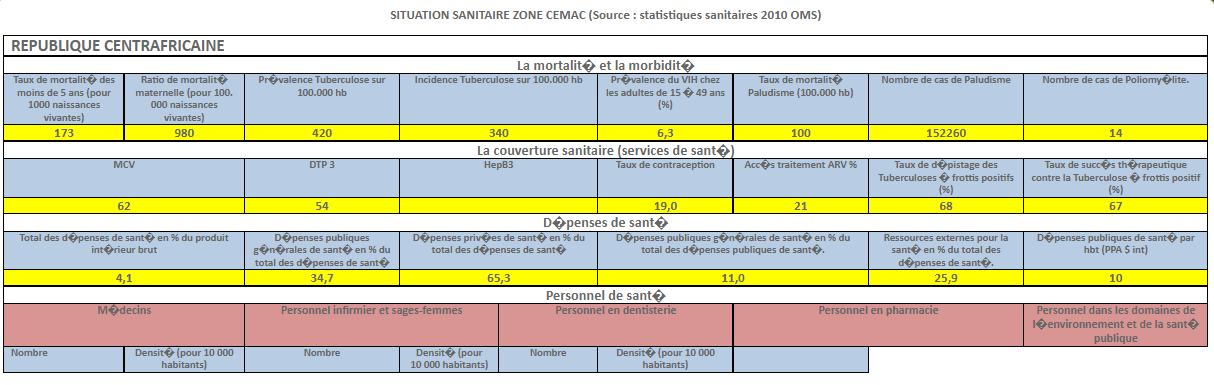
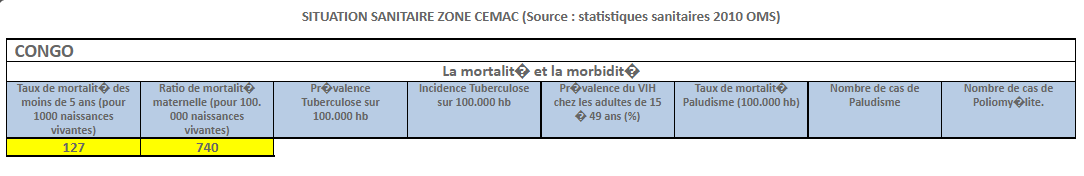
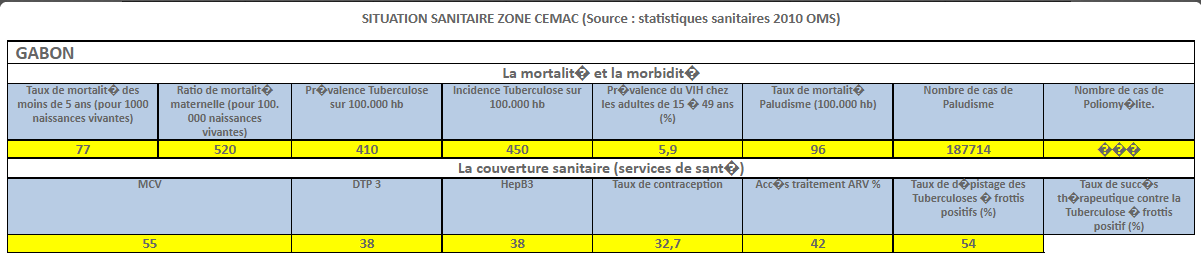
INTRODUCTION
Anxious to improve the health care of the populations of the countries of the sub-region, the Heads of State of CEMAC have mandated OCEAC, for more than a decade, to lead the process of harmonization of National Pharmaceutical Policies of Member States. In this context, these Heads of State adopted, in June 2013, a Common Pharmaceutical Policy (CPP) whose main purpose is to enable all populations to have access to safe, effective, good quality and affordable pharmaceutical products. lower cost. This PPC includes, among other various areas, the fight against counterfeit drugs and the illicit sale of drugs. Indeed, the current situation, at the global level in general and at the level of the Central African sub-region in particular, is marked by the rise of the scourges of the circulation of counterfeit drugs and illicit drug trafficking. A five (05) year sub-regional action plan to combat these scourges has been adopted by the political authorities and CEMAC bodies. The implementation of the PPC is thus done through multi-year action plans (PAP) responding to its various areas, including this plan to fight against counterfeit medicines. The activities of these PAPs are included in annual operational plans (POA) and implemented in collaboration with the countries, with the support of partners. In this context, many efforts have been made to date, but the fact remains that much remains to be done.
GENERAL OBJECTIVE AND EXPECTED RESULT OF THE PSR/HPPN
- Main objective: Lead the Member States towards the harmonization of their national pharmaceutical policies, on the basis of the Common Pharmaceutical Policy.
- Overall expected result: The pharmaceutical policies of the Member States are harmonized on the basis of the PPC.